Extension of the EU biocidal active substance review programme
The EU authorities have agreed to extend the review programme for existing active substances until 31 December 2030. The official text is yet to come.

As a reminder, the review programme has already been extended twice. Initially under the Biocide Product Directive (BDP), the evaluation of active substances was set to be finished on 14 May 2010. This duration was extended by 4 years until 14 May 2014. With the BPR, the deadline became 31 December 2024 (Article 89.1). To date less than 45% of the actives have been reviewed.
The extension of this deadline has an impact on current national authorisations under transitional measures valid until 31 December 2024 and actions may be required in some member states. This is the case in Belgium where the authorities have already informed transitional authorisation holders about renewal/prolongation dossier submission deadlines.
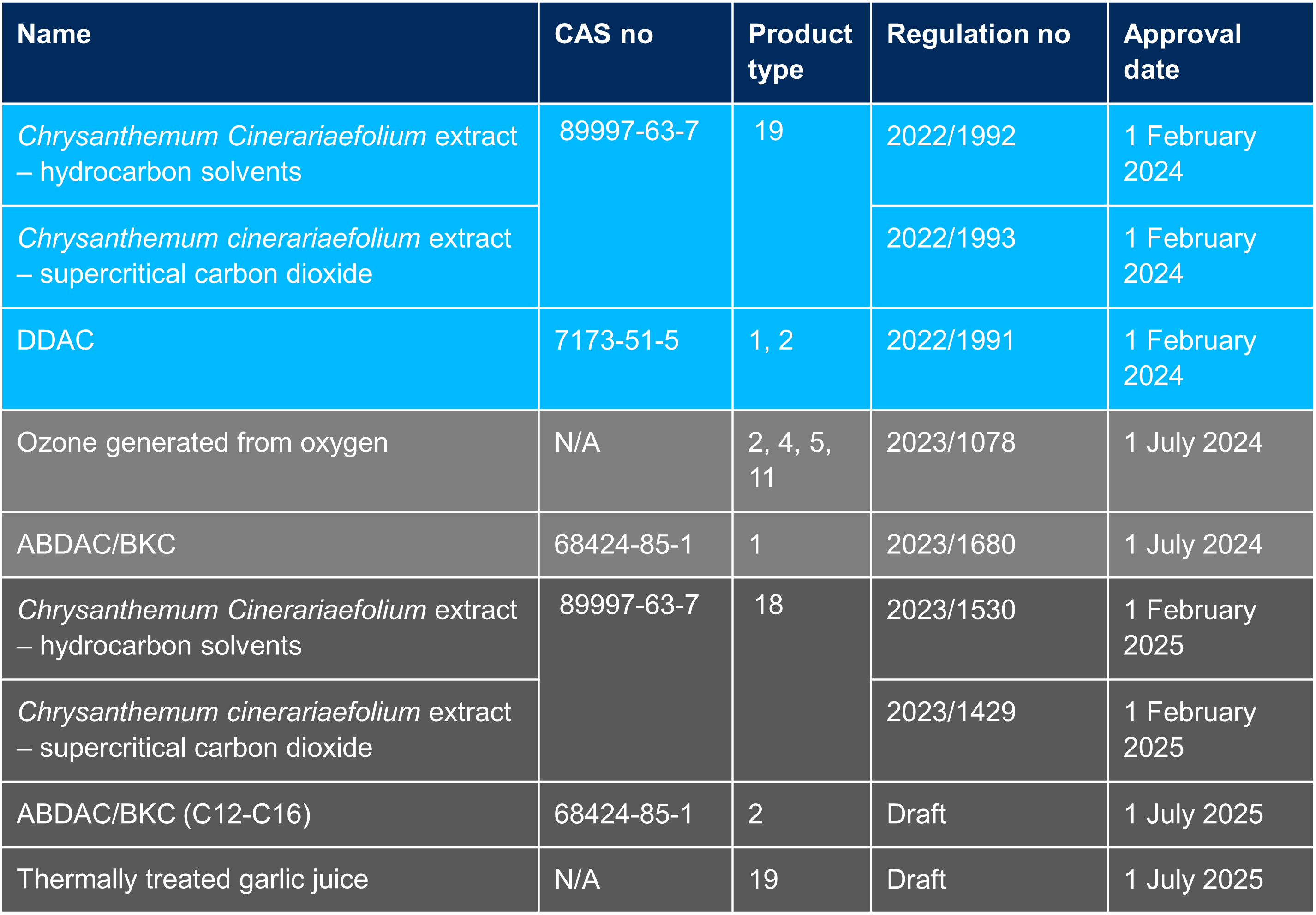
Upcoming active substance approval deadlines
TSG is ready to help you with the preparation of your BPR dossier for biocidal products based on the active substances in the table below. Remember to start early to ensure there is enough time for considering the new Biocidal Product Family guidance, as well as the shelf-life testing and seasonal efficacy testing that require planning.
Preparing SPC under IUCLID
Until now the Summary of Product Characteristics (SPC) – also known as the 'Identity Card' of the authorised biocide product/family – was prepared using the SPC Editor. This will change at the end of 2023 as IUCLID will need to be used to prepare SPCs. A new version of IUCLID including this change and other REACH updates is therefore live.
Get in touch
If you have any questions about preparing your BPR dossier, or testing and efficacy requirements, please do get in touch with our team at [email protected].
Alternatively, our regulatory consultants will be attending the upcoming Congrès Biocides (17-18 October, Lyon, France) and Biocides Europe 2023 (11-12 December, Vienna, Austria). Do reach out to [email protected] if you'd like to put your questions to them in person.